PK-A
ONLINE AMPOULES AND CARTRIDGES CONTAINER CLOSURE INTEGRITY TESTER
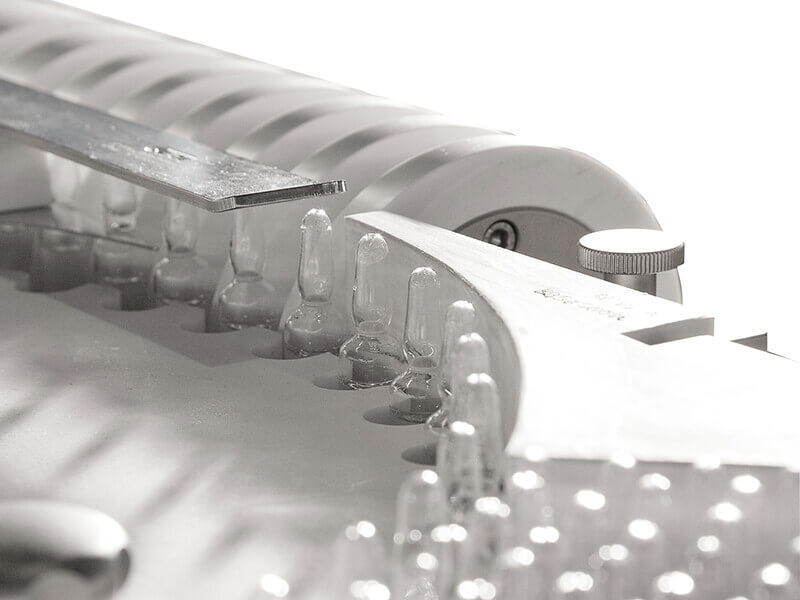
The Machine is designed for Non-Destructive Integrity Testing of Containers with pharmaceutical products at high production speed.
The Machine is suitable for 100% off-line testing.
The Measurement System comprises applying a pressure differential into an airtight Testing Group enclosing the Container (Patent No. 1225063 of 13-9-1988). The test objective is to detect Container leakages by measuring the reached pressure level as well as the pressure change over test time.
The Measurement System follows the approved industry standard “ASTM F2338-09 – Standard Test Method for Non-Destructive Detection of Leaks in Packages”.
The Test method is a Recognised Consensus Standard by the United States Food and Drug Administration (FDA), Center for Devices and Radiological Health (CDRH), effective March 31, 2006.